
Polarity of Molecules 11/18/14 Polar Molecules are molecules which have an uneven distribution of charge. One side of the molecule is negative while. - ppt download

Ozone in Polar (Acetonitrile) and Nonpolar (Carbon Tetrachloride) Organic Liquids: Optical Absorption, Solubility, and Stability - Ershov - 2022 - ChemistrySelect - Wiley Online Library
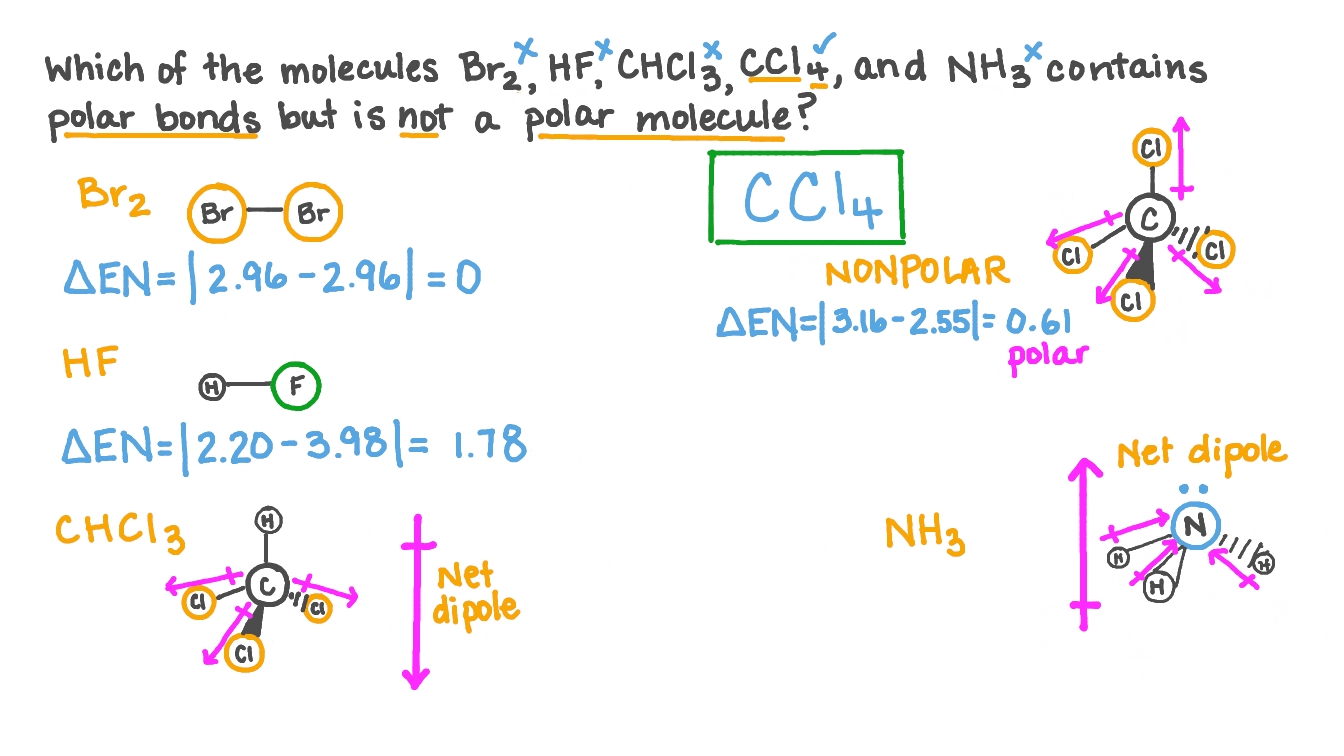
Question Video: Determining the Molecule That Contains Polar Bonds but Is Not a Polar Molecule | Nagwa

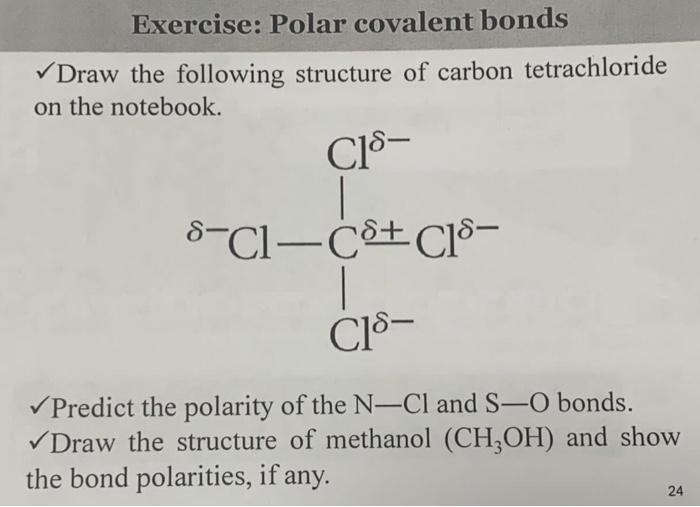
Draw the structure of carbon tetrachloride according to Lewis theory. What would be its associated molecular geometry? (a) tetrahedral (b) linear (c) trigonal pyramidal (d) bent | Homework.Study.com

Carbon tetrachloride CCl4 lewis dot structure, molecular geometry, polar or nonpolar, Bond angle | Molecular geometry, Molecular shapes, Molecular

OneClass: Be sure to answer all parts The four bonds of carbon tetrachloride (CCI4) are polar, but th...


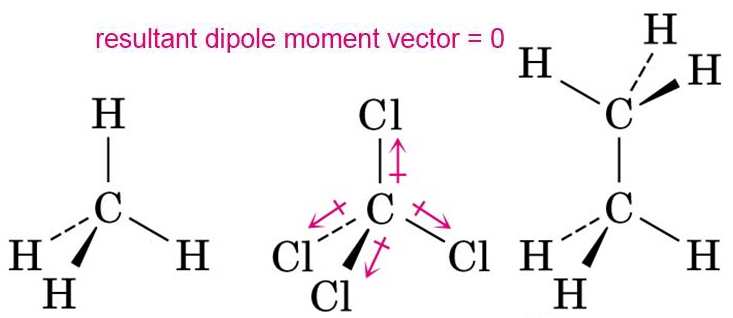


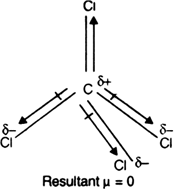



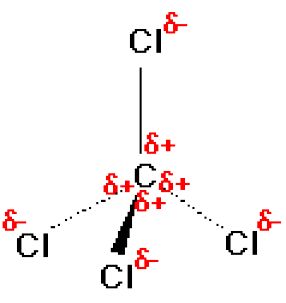


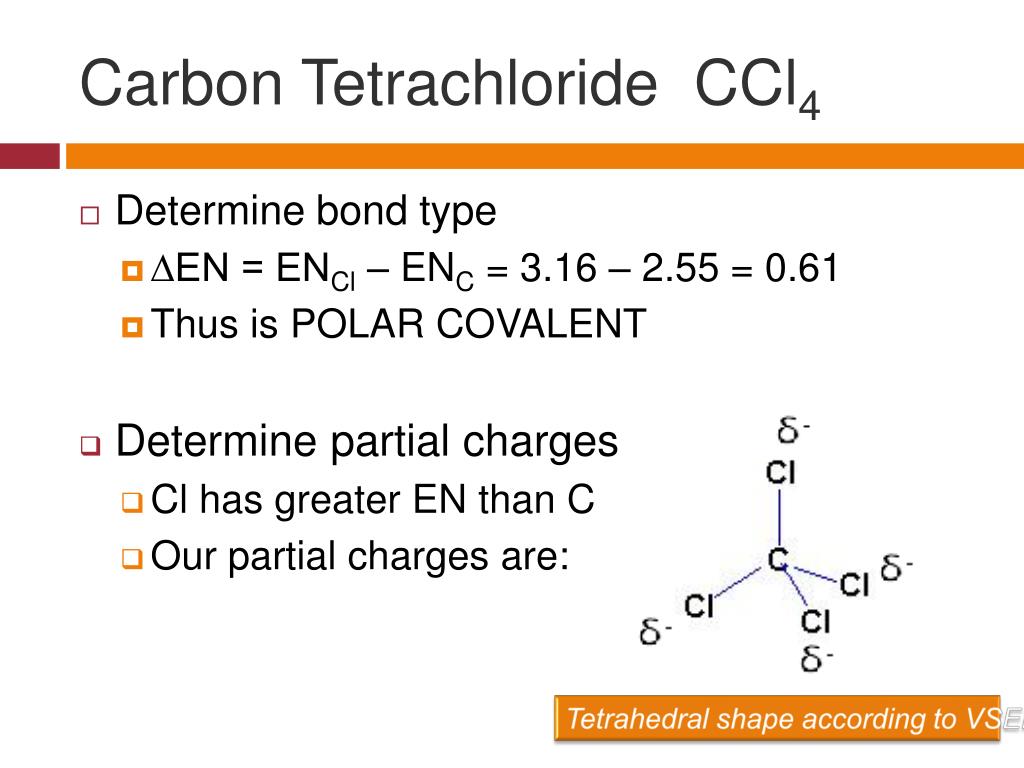

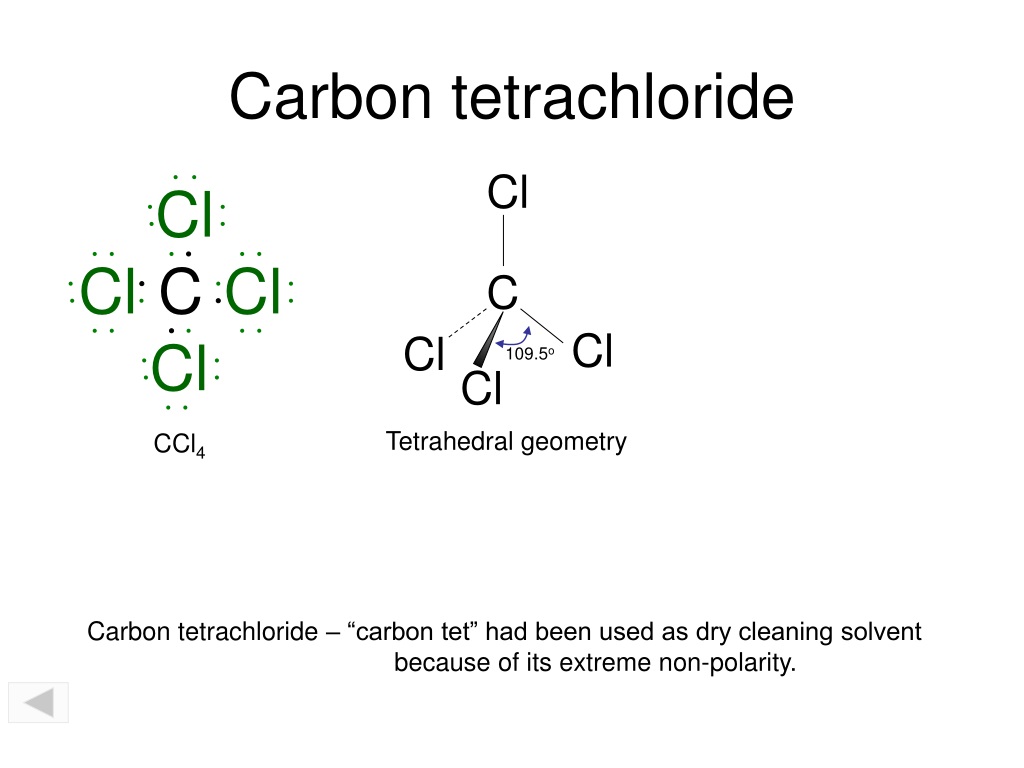
![Is \\[CC{l_4}\\] polar or nonpolar? Is \\[CC{l_4}\\] polar or nonpolar?](https://www.vedantu.com/question-sets/dbf3f5ee-35e7-43b7-b8a3-cfe63dfd520e2097468885289678636.png)