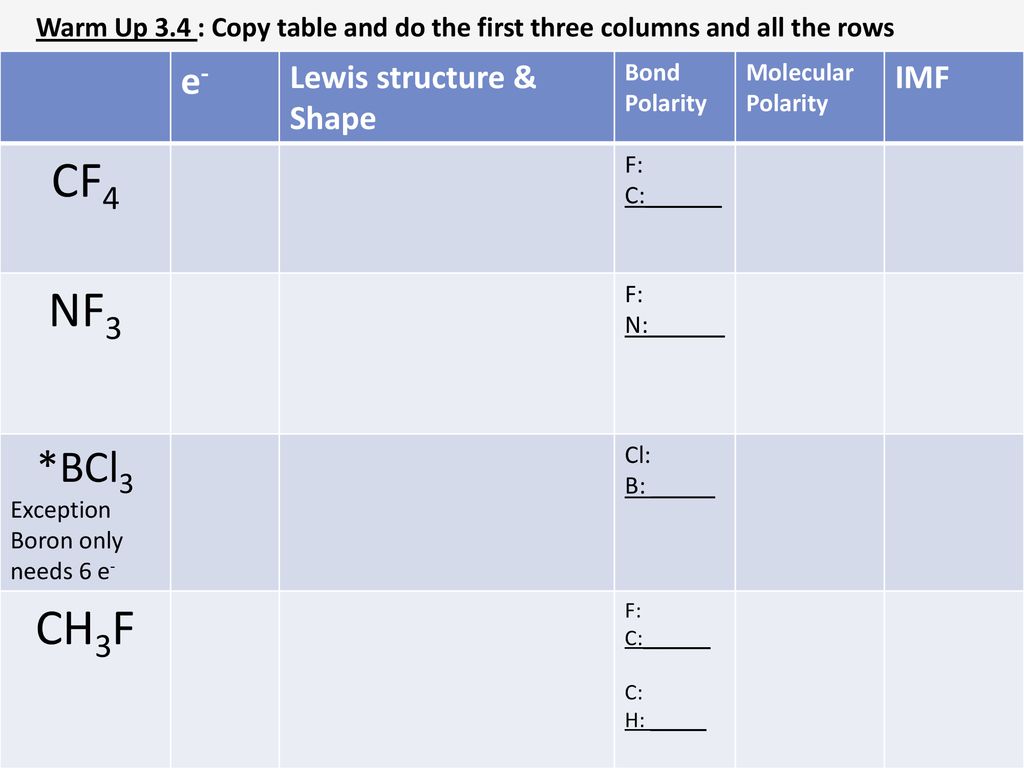
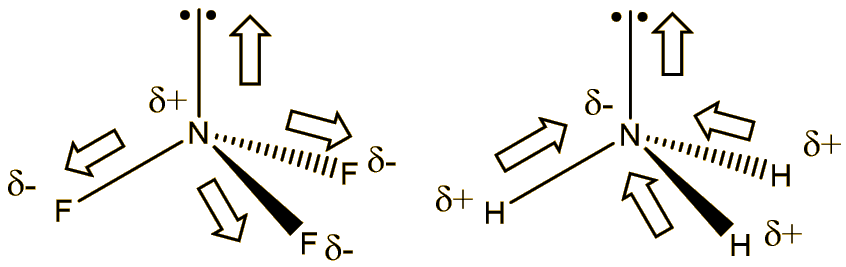
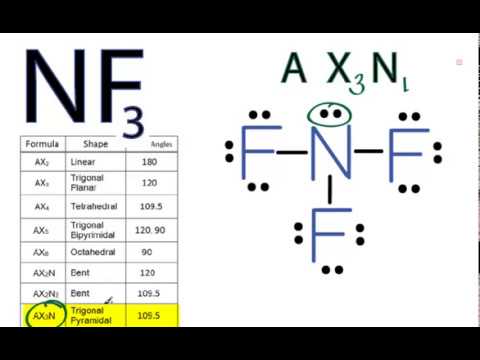
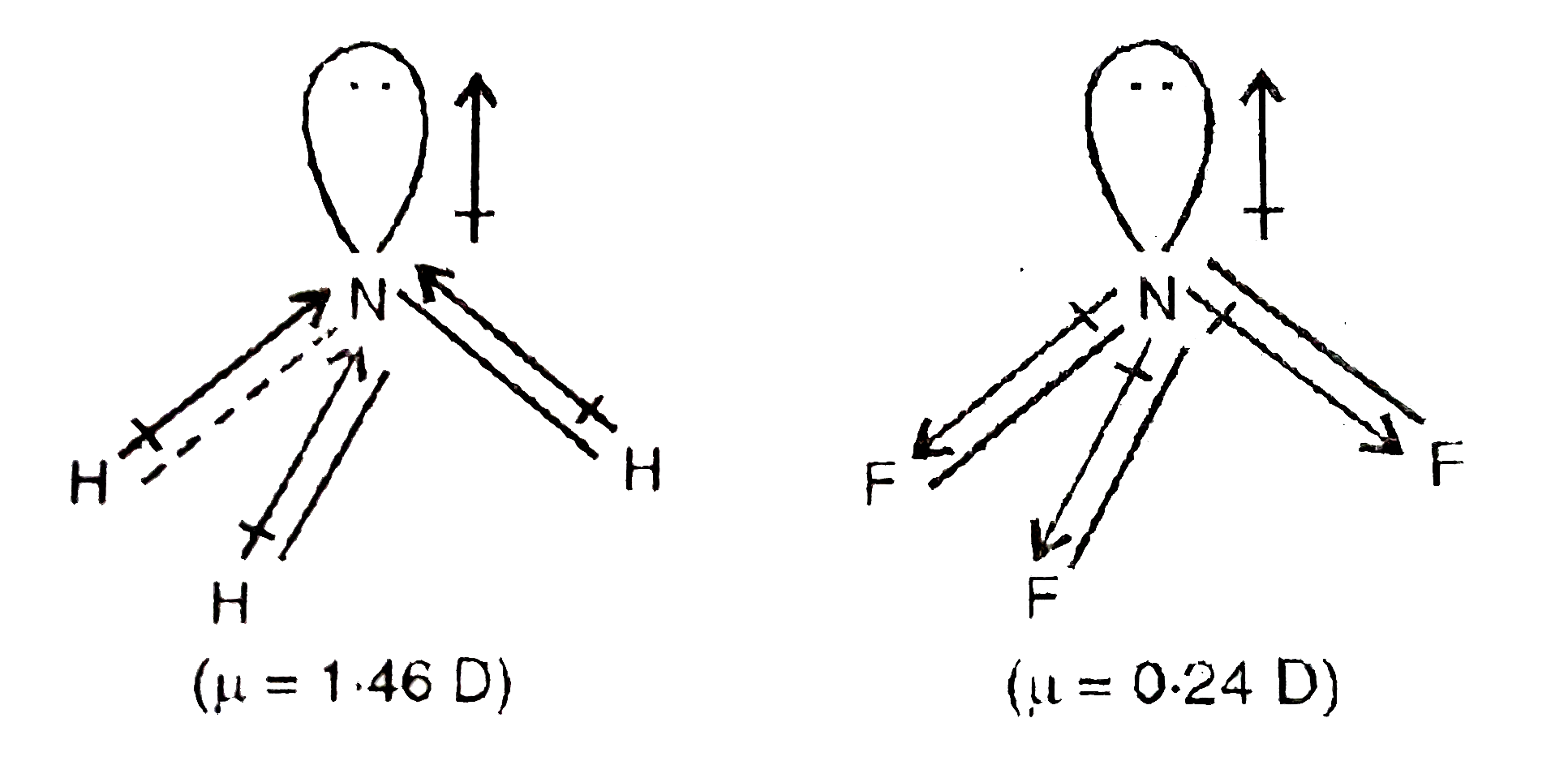
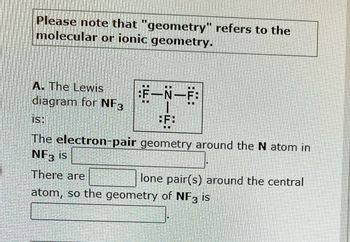
write lewis structures of nf3. what is the electronic and molecular geometry? is the molecule polar or - brainly.com
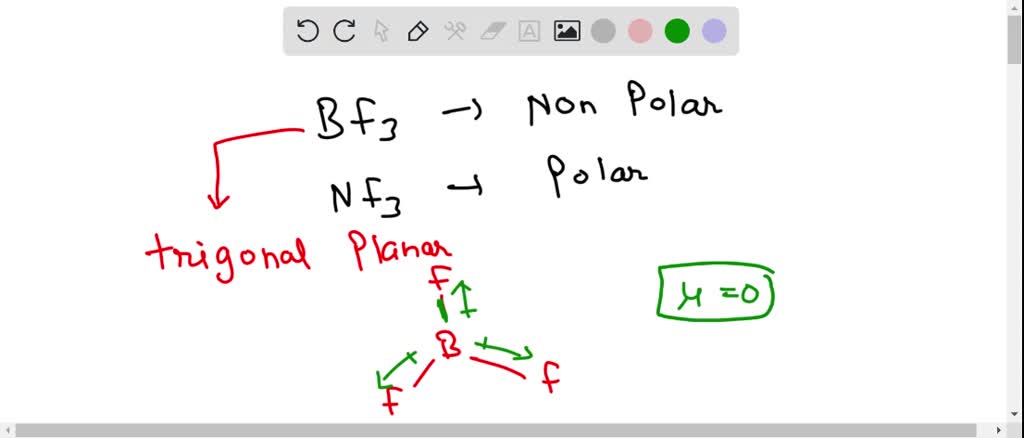
SOLVED: 41. The BF3 molecule is nonpolar, whereas the NF3 molecule is polar. Which of the following statements accounts for the difference in polarity of the two molecules? In NF3, each F

NF3 Lewis Structure (Nitrogen Trifluoride) | NF3 Lewis Structure (Nitrogen Trifluoride) Were you searching for a short yet detailed video on NF3 Lewis Structure? If yes then we have got you. Today...

In a polar nitrogen trifluoride, NF3 molecule, nitrogen and fluorine atoms share electrons. The fluorine - brainly.com
Both BF3 and NF3 are covalent compounds but NF3 is a polar compound while BF3 is non-polar. How can you explain it? - Quora