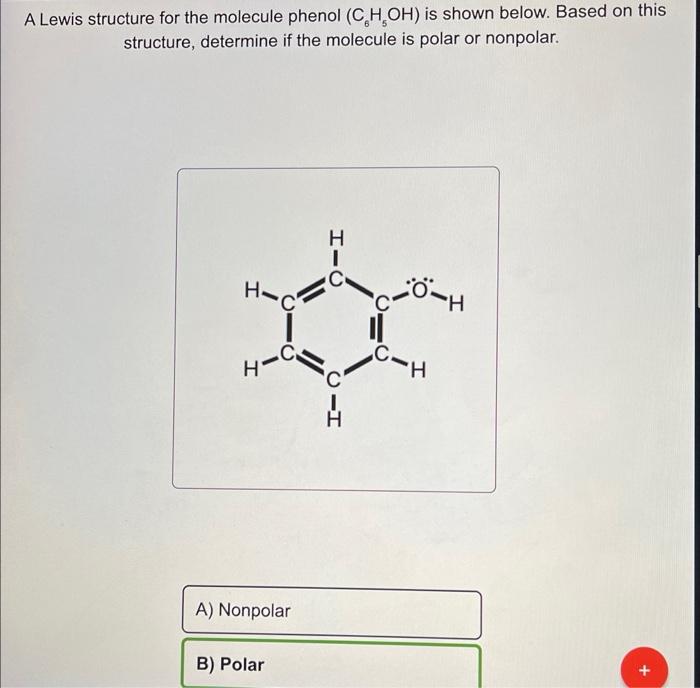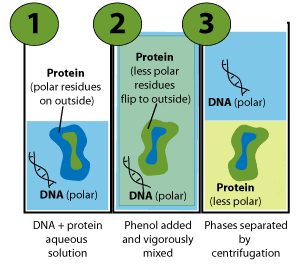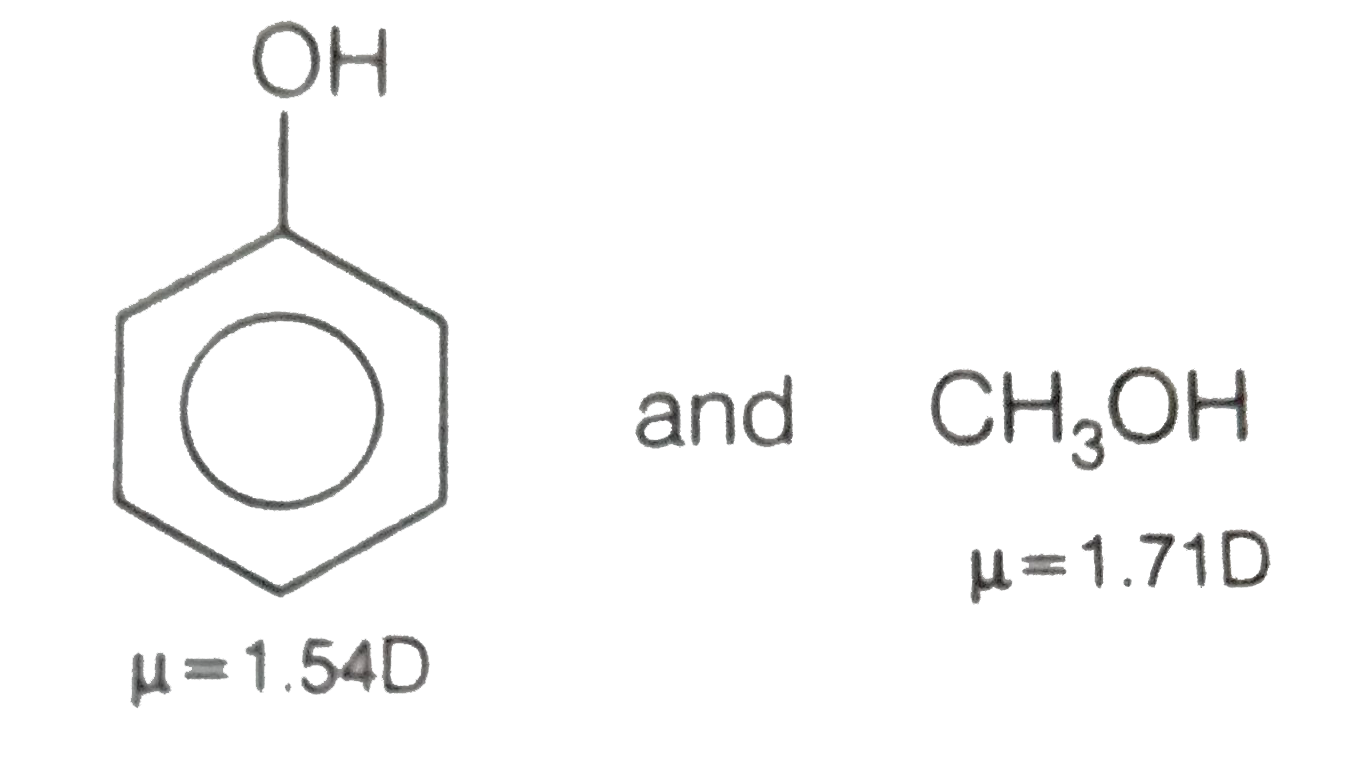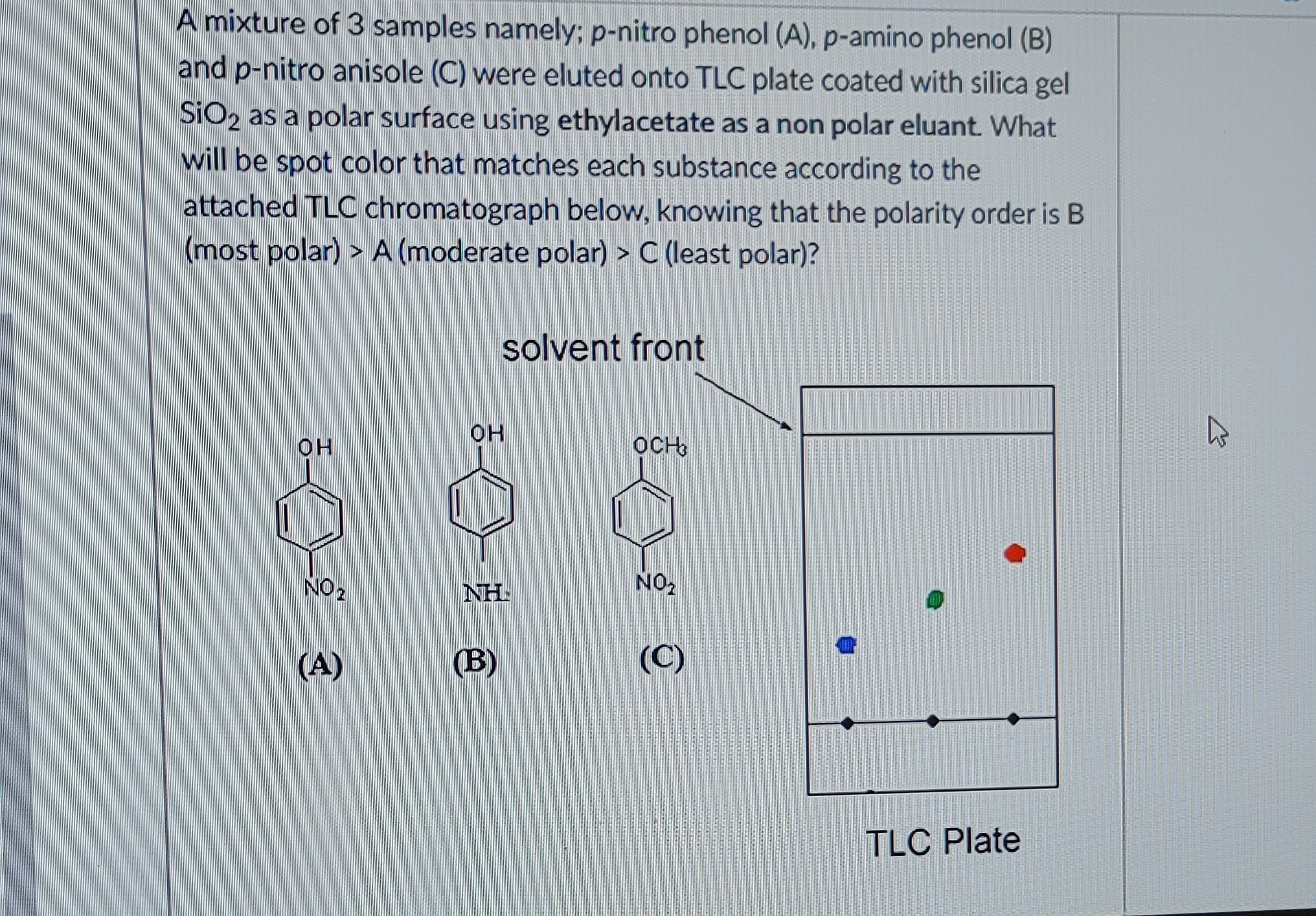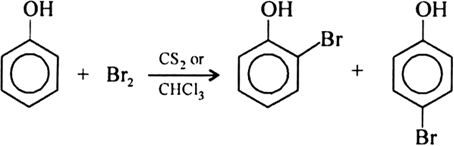
What happens when phenol reacts with bromine in solvent of low polarity like CS2or CHCl3 at low temperature? Give a mechanism for the reaction. - Zigya

Which would you expect to be more polar: phenol or parachlorophenol? Which compound would you expect to have the higher melting point? Explain. | Homework.Study.com
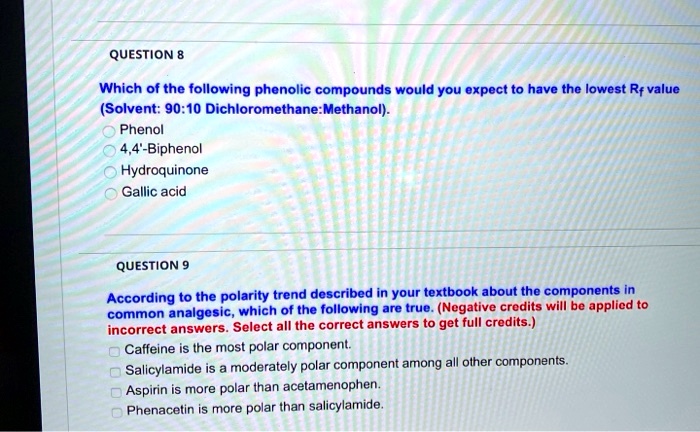
SOLVED: QUESTION 8 Which of the following phenolic compounds would you expect to have the lowest Rf value (Solvent: 90:10 Dichloromethane:Methanol)? A. Phenol B. 4,4'-Biphenol C. Hydroquinone D. Gallic acid QUESTION 9

What happens when phenol reacts with bromine in solvent of low polarity like CS2or CHCl3 at low temperature? Give a mechanism for the reaction. - Zigya

Direct Irradiation of Phenol and Para-Substituted Phenols with a Laser Pulse (266 nm) in Homogeneous and Micro-heterogeneous Media. A Time-Resolved Spectroscopy Study | The Journal of Organic Chemistry

Phenol hydrogen bonding physical chemical properties electrophilic substitution chlorine bromine nitric acid acidity of phenols uses chlorophenols phenyl phenolic esters advanced A level organic chemistry revision notes doc brown

Table 1 from Sorption of polar and nonpolar aromatic organic contaminants by plant cuticular materials: role of polarity and accessibility. | Semantic Scholar

Arrange the following solvents in order of increasing polarity: a) ethanol b) ethyl acetate c) petroleum ether d) toluene e) acetone | Homework.Study.com






![Odia] Give the order of polarity of alcohol, phenol and ether. Odia] Give the order of polarity of alcohol, phenol and ether.](https://static.doubtnut.com/ss/web-overlay-thumb/9347040.webp)