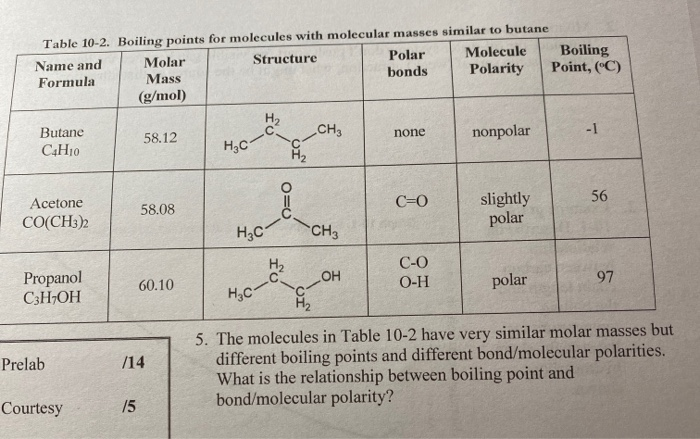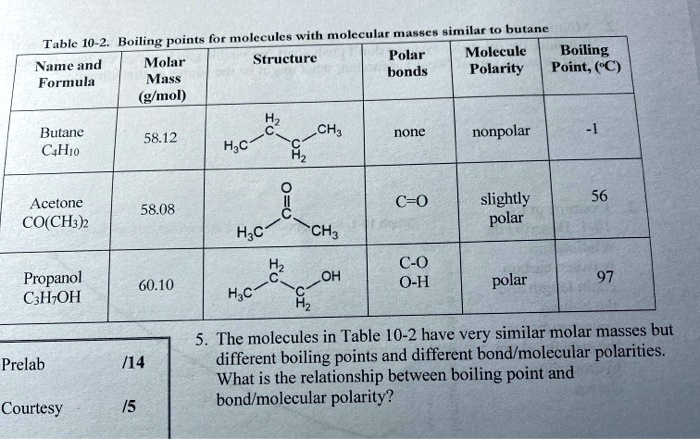
SOLVED: Text: with Heptane Aaee Similar to butane Table 10-2- Boiling points for molecules Polar Molecule Boiling Molar Structure Name and bonds Polarity Point; (C) Formula Mass (g/mol) Butane C4H10 CH3 none

Diethyl ether has a much higher boiling point than butane despite having a higher molecular weight. Explain why this is the case, making reference to the molecular structures of both compounds.

Arrange the following solvents in order of increasing polarity: a) ethanol b) ethyl acetate c) petroleum ether d) toluene e) acetone | Homework.Study.com